Google’s crack at a quantum computer is a bid to change computing forever.
- By Tom Simonite on September 3, 2014
Why It Matters
Practical quantum computers could solve problems that would take conventional machines millions of years.
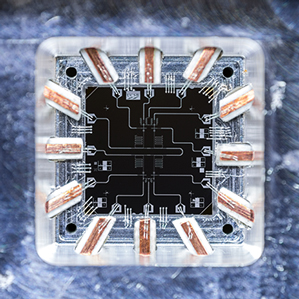
Quantum core: Techniques developed at the University of California, Santa Barbara, to build this device, known as a qubit, will be used to try to build a working quantum computer at Google.
Google is about to begin designing and building hardware for a quantum computer, a type of machine that can exploit quantum physics to solve problems that would take a conventional computer millions of years.
Since 2009, Google has been working with controversial startup D-Wave Systems, which claims to make “the first commercial quantum computer.” And last year Google purchased one of D-Wave’s machines. But independent tests published earlier this year found no evidence that D-Wave’s computer uses quantum physics to solve problems more efficiently than a conventional machine.
Now John Martinis, a professor at University of California, Santa Barbara, has joined Google to establish a new quantum hardware lab near the university. He will try to make his own versions of the kind of chip inside a D-Wave machine.
Martinis has spent more than a decade working on a more proven approach to quantum computing, and built some of the largest, most error-free systems of qubits, the basic building blocks that encode information in a quantum computer.
“We would like to rethink the design and make the qubits in a different way,” says Martinis of his effort to improve on D-Wave’s hardware. “We think there’s an opportunity in the way we build our qubits to improve the machine.” Martinis has taken a joint position with Google and UCSB that will allow him to continue his own research at the university.
Quantum computers could be immensely faster than any existing computer at certain problems. That’s because qubits working together can use the quirks of quantum mechanics to quickly discard incorrect paths to a solution and home in on the correct one. However, qubits are tricky to operate because quantum states are so delicate.
Chris Monroe, a professor who leads a quantum computing lab at the University of Maryland, welcomed the news that one of the leading lights in the field was going to work on the question of whether designs like D-Wave’s can be useful. “I think this is a great development to have legitimate researchers give it a try,” he says.
Since showing off its first machine in 2007, D-Wave has irritated academic researchers by making claims for its computers without providing the evidence its critics say is needed to back them up. However, the company has attracted over $140 million in funding and sold several of its machines (see “The CIA and Jeff Bezos Bet on Quantum Computing”).
There is no question that D-Wave’s machine can perform calculations. And research published in 2011 showed that the machine’s chip harbors the right kind of quantum physics needed for quantum computing. But evidence is lacking that it uses that physics in the way needed to unlock the huge speedups promised by a quantum computer. It could be solving problems using only ordinary physics.
Martinis’s previous work has been focused on the conventional approach to quantum computing. He set a new milestone in the field this April, when his lab announced that it could operate five qubits together with relatively low error rates. Larger systems of such qubits could be configured to run just about any kind of algorithm depending on the problem at hand, much like a conventional computer. To be useful, a quantum computer would probably need to be built with tens of thousands of qubits or more.
The chip at the heart of D-Wave’s latest machine has 512 qubits, but they are wired into a different, more limited, component known as a quantum annealer. It can only run a specific algorithm used for a specific kind of problem that requires selecting the best option in a situation with many competing requirements—for example, determining the most efficient delivery route around a city.
Martinis was a coauthor on a paper published in Science earlier this year that took the most rigorous independent look at a D-Wave machine yet. It concluded that in the tests run on the computer, there was “no evidence of quantum speedup.” Without that, critics say, D-Wave is nothing more than an overhyped, and rather weird, conventional computer. The company counters that the tests of its machine involved the wrong kind of problems to demonstrate its benefits.
Martinis’s work on D-Wave’s machine led him into talks with Google, and to his new position. Theory and simulation suggest that it might be possible for annealers to deliver quantum speedups, and he considers it an open question. “There’s some really interesting science that people are trying to figure out,” he says.
Martinis thinks his technology for fabricating qubits could make better quantum annealers. Specifically, he hopes to make one whose qubits can more stably maintain a quantum state known as a superposition—effectively both 0 and 1 at the same time. The qubits of D-Wave’s machine can maintain superpositions for periods lasting only nanoseconds. Martinis has built qubits that can do that for as long as 30 microseconds, he says.
Martinis makes his qubits from aluminum circuits built on sapphire wafers and chills them to 20 millikelvin—a fraction above absolute zero—so that they become superconducting. D-Wave’s chip requires similar cooling to operate, but has circuits made from a superconducting material called niobium, on top of silicon wafers. Martinis is in the process of switching to making his own qubits on silicon, and believes certain electrical insulator materials used in D-Wave’s chips may be limiting its performance.
However, Google has not given up on D-Wave. In an online statement, the leader of Google’s quantum research said that the two companies will continue to work together, and that Google’s D-Wave computer will be upgraded with a new 1,000 qubit processor when it becomes available.















