
Zinc, in commerce also spelter, is a chemical element with symbol Znand atomic number 30. It is the first element of group 12 of theperiodic table. In some respects zinc is chemically similar tomagnesium: its ion is of similar size and its only common oxidation state is +2. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite(zinc blende), a zinc sulfide mineral. The largest mineable amounts are found in Australia, Asia, and the United States. Zinc production includes froth flotation of the ore, roasting, and final extraction usingelectricity (electrowinning). Brass, which is an alloy of copper and zinc, has been used since at least the 10th century BC in Judea and by the 7th century BC in Ancient Greece. Zinc metal was not produced on a large scale until the 12th century in India and was unknown to Europe until the end of the 16th century. The mines of Rajasthan have given definite evidence of zinc production going back to the 6th century BC. To date, the oldest evidence of pure zinc comes from Zawar, in Rajasthan, as early as the 9th century AD when a distillation process was employed to make pure zinc. Alchemists burned zinc in air to form what they called "philosopher's wool" or "white snow". The element was probably named by the alchemist Paracelsus after the German word Zinke. German chemist Andreas Sigismund Marggraf is credited with discovering pure metallic zinc in 1746. Work by Luigi Galvani and Alessandro Volta uncovered the electrochemical properties of zinc by 1800. Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Other applications are in batteries, small non-structural castings, and alloys, such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory. Zinc is an essential mineral perceived by the public today as being of "exceptional biologic and public health importance", especially regarding prenatal and postnatal development. Zinc deficiency affects about two billion people in the developing world and is associated with many diseases. In children it causes growth retardation, delayed sexual maturation, infection susceptibility, and diarrhea. Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as alcohol dehydrogenase in humans. Consumption of excess zinc can causeataxia, lethargy and copper deficiency. Zinc is an essential trace element for humans and other animals, for plants and for microorganisms. Zinc is found in nearly 100 specificenzymes (other sources say 300), serves as structural ions in transcription factors and is stored and transferred in metallothioneins. It is "typically the second most abundant transition metal in organisms" after iron and it is the only metal which appears in all enzyme classes. In proteins, Zn ions are often coordinated to the amino acid side chains of aspartic acid, glutamic acid, cysteine and histidine. The theoretical and computational description of this zinc binding in proteins (as well as that of other transition metals) is difficult. There are 2-4 grams of zinc distributed throughout the human body. Most zinc is in the brain, muscle, bones, kidney, and liver, with the highest concentrations in the prostate and parts of the eye. Semen is particularly rich in zinc, which is a key factor in prostate gland function and reproductive organgrowth. In humans, zinc plays "ubiquitous biological roles". It interacts with "a wide range of organic ligands",and has roles in the metabolism of RNA and DNA,signal transduction, and gene expression. It also regulates apoptosis. A 2006 study estimated that about 10% of human proteins (2800) potentially bind zinc, in addition to hundreds which transport and traffic zinc; a similar in silico study in the plant Arabidopsis thaliana found 2367 zinc-related proteins. In the brain, zinc is stored in specific synaptic vesicles by glutamatergicneurons and can "modulate brain excitability". It plays a key role in synaptic plasticity and so in learning. However, it has been called "the brain's dark horse because it also can be a neurotoxin, suggesting zinc homeostasis plays a critical role in normal functioning of the brain and central nervous system. Enzymes 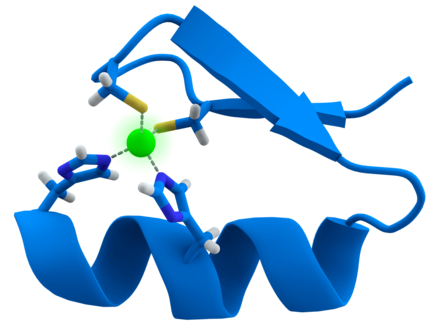
Zinc fingers help read DNA sequences. Zinc is an efficient Lewis acid, making it a useful catalytic agent in hydroxylationand other enzymatic reactions. The metal also has a flexible coordination geometry, which allows proteins using it to rapidly shift conformations to perform biological reactions. Two examples of zinc-containing enzymes arecarbonic anhydrase and carboxypeptidase, which are vital to the processes ofcarbon dioxide () regulation and digestion of proteins, respectively. In vertebrate blood, carbonic anhydrase converts into bicarbonate and the same enzyme transforms the bicarbonate back into for exhalation through the lungs. Without this enzyme, this conversion would occur about one million times slower at the normal blood pH of 7 or would require a pH of 10 or more. The non-related β-carbonic anhydrase is required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and alcoholic fermentation. Carboxypeptidase cleaves peptide linkages during digestion of proteins. Acoordinate covalent bond is formed between the terminal peptide and a C=O group attached to zinc, which gives the carbon a positive charge. This helps to create a hydrophobic pocket on the enzyme near the zinc, which attracts the non-polar part of the protein being digested Other proteins Zinc serves a purely structural role in zinc fingers, twists and clusters. Zinc fingers form parts of some transcription factors, which are proteins that recognize DNA base sequences during the replication and transcription of DNA. Each of the nine or ten ions in a zinc finger helps maintain the finger's structure by coordinately binding to four amino acids in the transcription factor. The transcription factor wraps around the DNA helix and uses its fingers to accurately bind to the DNA sequence. In blood plasma, zinc is bound to and transported by albumin (60%, low-affinity) and transferrin (10%). Because transferrin also transports iron, excessive iron reduces zinc absorption, and vice versa. A similar antagonism exists with copper. The concentration of zinc in blood plasma stays relatively constant regardless of zinc intake. Cells in the salivary gland, prostate, immune system and intestine use zinc signaling as one way to communicate with other cells. Zinc may be held in metallothionein reserves within microorganisms or in the intestines or liver of animals. Metallothionein in intestinal cells is capable of adjusting absorption of zinc by 15–40%. However, inadequate or excessive zinc intake can be harmful; excess zinc particularly impairs copper absorption because metallothionein absorbs both metals. Dietary intake 
Foods & spices containing zinc In the U.S., the Recommended Dietary Allowance (RDA) is 8 mg/day for women and 11 mg/day for men. Median intake in the U.S. around 2000 was 9 mg/day for women and 14 mg/day in men. Oysters, lobster and red meats, especially beef, lamb and liver have some of the highest concentrations of zinc in food. Zinc supplements should only be ingested when there is zinc deficiency or increased zinc necessity (e.g. after surgeries, traumata or burns) Persistent intake of high doses of zinc can cause copper deficiency. The concentration of zinc in plants varies based on levels of the element in soil. When there is adequate zinc in the soil, the food plants that contain the most zinc are wheat (germ and bran) and various seeds (sesame, poppy, alfalfa, celery,mustard). Zinc is also found in beans, nuts, almonds, whole grains, pumpkin seeds, sunflower seeds and blackcurrant. Other sources include fortified food and dietary supplements, which come in various forms. A 1998 review concluded that zinc oxide, one of the most common supplements, and zinc carbonate are nearly insoluble and poorly absorbed in the body. This review cited studies which found low plasma zinc concentrations after zinc oxide and zinc carbonate were consumed compared with those seen after consumption of zinc acetate and sulfate salts. However, harmful excessive supplementation is a problem among the relatively affluent, and should probably not exceed 20 mg/day in healthy people, although the U.S. National Research Council set a Tolerable Upper Intake of 40 mg/day. For fortification, however, a 2003 review recommended zinc oxide in cereals as cheap, stable, and as easily absorbed as more expensive forms. A 2005 study found that various compounds of zinc, including oxide and sulfate, did not show statistically significant differences in absorption when added as fortificants to maize tortillas. A 1987 study found that zinc picolinate was better absorbed than zinc gluconate or zinc citrate. However, a study published in 2008 determined that zinc glycinate is the best absorbed of the four dietary supplement types available. Deficiency Main article: Zinc deficiency Zinc deficiency is usually due to insufficient dietary intake, but can be associated with malabsorption, acrodermatitis enteropathica, chronic liver disease, chronic renal disease, sickle cell disease, diabetes, malignancy, and other chronic illnesses. Groups at risk for zinc deficiency include the elderly, children in developing countries, and those with renal insufficiency. Symptoms of mild zinc deficiency are diverse. Clinical outcomes include depressed growth, diarrhea, impotence and delayed sexual maturation, alopecia, eye and skin lesions, impaired appetite, altered cognition, impaired host defense properties, defects in carbohydrate utilization, and reproductive teratogenesis. Mild zinc deficiency depresses immunity, although excessive zinc does also. Animals with a diet deficient in zinc require twice as much food in order to attain the same weight gain as animals given sufficient zinc. Despite some concerns, western vegetarians and vegans have not been found to suffer from overt zinc deficiencies any more than meat-eaters.200 Major plant sources of zinc include cooked dried beans, sea vegetables, fortified cereals, soyfoods, nuts, peas, and seeds However, phytates in many whole-grains and fiber in many foods may interfere with zinc absorption and marginal zinc intake has poorly understood effects. The zinc chelator phytate, found in seeds and cereal bran, can contribute to zinc malabsorption.7 There is some evidence to suggest that more than the US RDA (15 mg) of zinc daily may be needed in those whose diet is high in phytates, such as some vegetarians. These considerations must be balanced against the fact that there is a paucity of adequate zinc biomarkers, and the most widely used indicator, plasma zinc, has poor sensitivity and specificity. Diagnosing zinc deficiency is a persistent challenge. Nearly two billion people in the developing world are deficient in zinc. In children it causes an increase in infection and diarrhea, contributing to the death of about 800,000 children worldwide per year. The World Health Organization advocates zinc supplementation for severe malnutrition and diarrhea. Zinc supplements help prevent disease and reduce mortality, especially among children with low birth weight or stunted growth. However, zinc supplements should not be administered alone, because many in the developing world have several deficiencies, and zinc interacts with other micronutrients. http://www.wikiwand.com/en/Zinc |
















